CASE REPORT | https://doi.org/10.5005/jp-journals-10034-1098 |
Indispensable Role of Transesophageal Echocardiography in Elucidating the Mechanism of Bioprosthetic Heart Valve Dysfunction Causing Severe Regurgitation: A Case Report
1–5Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Corresponding Author: Bhupesh Kumar, Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India, Phone: +91 9914209511, e-mail: bhupeshkr@yahoo.com
How to cite this article Damodaran S, Ganesan R, Gourav KP, et al. Indispensable Role of Transesophageal Echocardiography in Elucidating the Mechanism of Bioprosthetic Heart Valve Dysfunction Causing Severe Regurgitation: A Case Report. J Perioper Echocardiogr 2019;7(1):15–17.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Transesophageal echocardiography (TEE) is invaluable in identifying the exact mechanism of prosthetic valve dysfunction (PVD), which may help in the proper management of patient. Here, we report a case of severe mitral regurgitation due to flail mitral valve leaflet resulting from torn bioprosthetic cusp where TEE was instrumental in demonstrating the exact mechanism of valve dysfunction.
Keywords: Bioprosthetic heart valves, Flail leaflet, Transesophageal echocardiography.
INTRODUCTION
Prosthetic valve dysfunction (PVD) is a serious complication with reported incidence of 0.1–6% per patient year.1 Exogenous causes of PVD causing regurgitation include excessive pannus overgrowth, residual chordae tendineae stuck in the prosthetic sewing ring, extremely long residual papillary muscles in the left ventricle, or calcified tissues under the prosthesis hampering leaflet mobility.2,3 The endogenous cause of PVD causing regurgitation such as cusp tear or perforation is rare but often warrants redo valve replacement surgery. The exact knowledge of the mechanism of PVD is essential to decide a therapeutic management plan. Although transthoracic echocardiography (TTE) is the noninvasive diagnostic modality of choice for the initial assessment of prosthetic valve function, transesophageal echocardiography (TEE) can identify the precise mechanism of PVD. We present a case of Hancock porcine valve dysfunction causing severe regurgitation due to flail motion of the torn cusp into the left atrium.
CASE DESCRIPTION
A 55-year-old male patient was admitted in our hospital with complaints of dyspnea on exertion of New York Heart Association class III. His native mitral valve was replaced with 29 mm Hancock bioprosthetic valve 8 years back for rheumatic mitral valve disease. He had no other associated comorbid illness or history suggestive of infective endocarditis (IE). Auscultation of chest revealed grade IV/VI pansystolic murmur. Chest X-ray showed bilateral lung congestion. Preoperative TTE showed thickened prosthetic valve leaflets with severe mitral regurgitation (Fig. 1 and Video 1) and a moderate tricuspid regurgitation. Two-dimensional TEE performed after induction of anesthesia in the operating room revealed a tear in one of the cusps causing flail motion of the leaflet into the left atrium resulting in posterior-directed wall hugging jet (Figs 2 and 3 and Videos 2 and 3). Left ventricular ejection fraction was 50% by modified Simpson’s biplane method. Three-dimensional (3D) echocardiography confirmed the flail motion of the cusp due to torn anterior cusp and thickening of commissural region (Fig. 4 and Video 4). No mass or thrombus was visualized on the prosthetic valve. The 3D color Doppler confirms regurgitant jet originating from the torn anterior cusp of the prosthesis (Fig. 5). Surgically extracted prosthetic valve also confirms torn segments of bioprosthetic valve (type I) and calcification in the commissural region (Fig. 6). Patient underwent redo mitral valve replacement with 27 mm St. Jude’s metallic valve using cardiopulmonary bypass (CPB). Transesophageal echocardiography examination performed after termination of CPB in the operating room showed mean gradient of 3 mm Hg across the metallic prosthetic valve with no para-valvular leak. Patient had uneventful postoperative course and was discharged from hospital on 10th postoperative day.
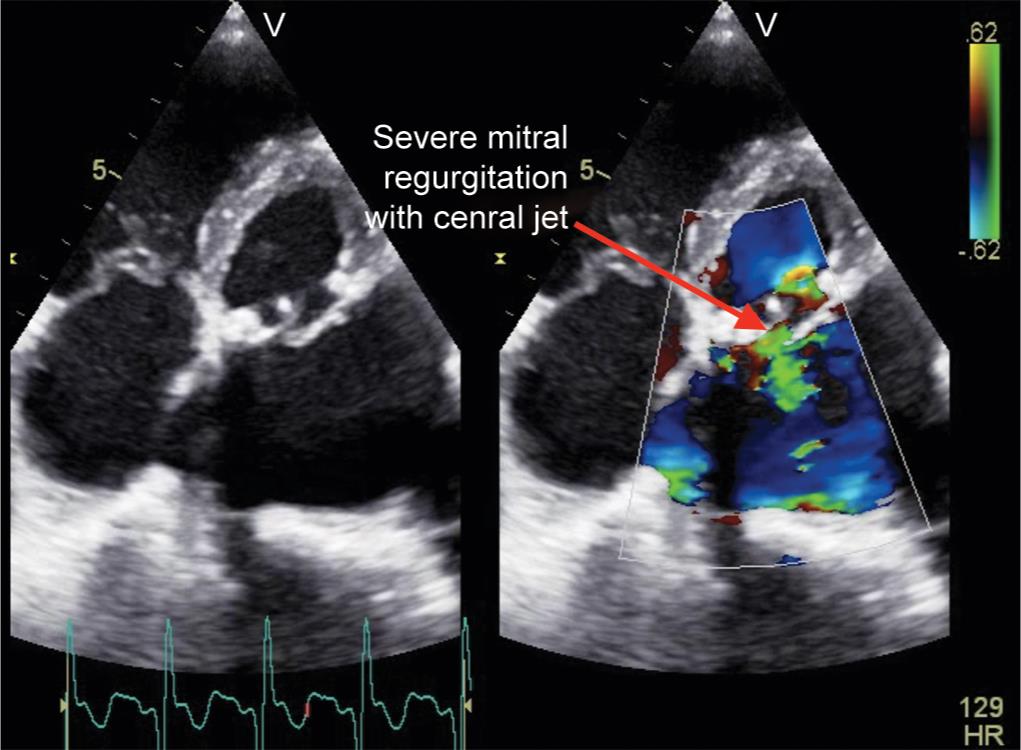
Fig. 1: Transthoracic four-chamber view with color doppler showing severe mitral regurgitation

Fig. 2: Midesophageal four-chamber view showing flail motion of torn bioprosthetic leaflet
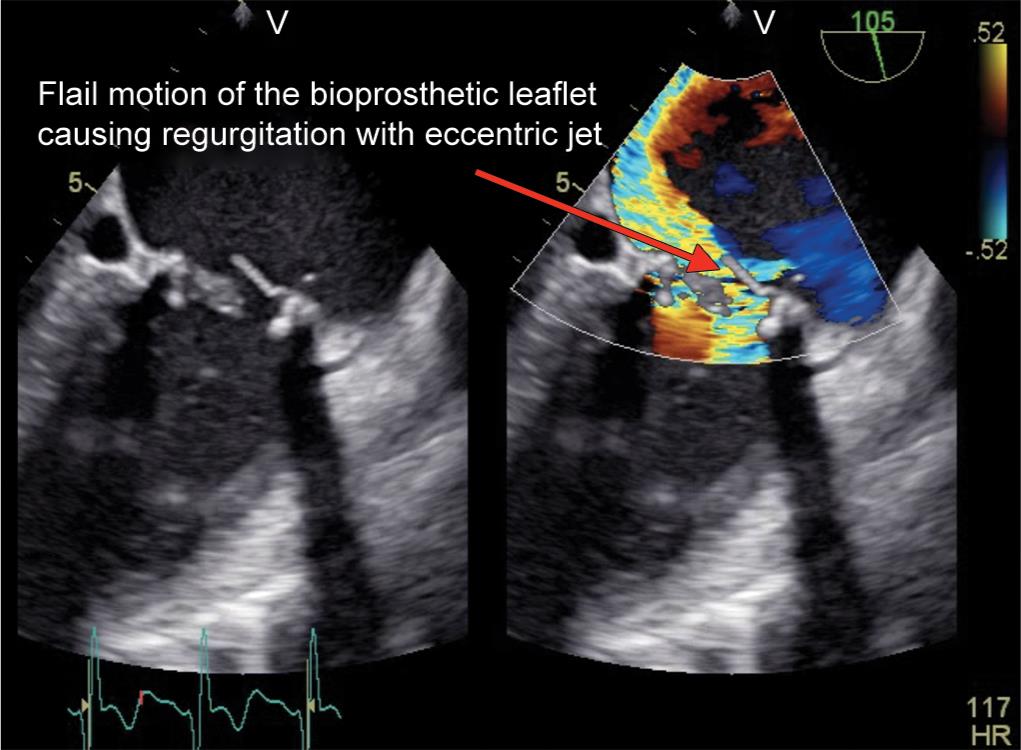
Fig. 3: Midesophageal two-chamber view with color doppler showing eccentric jet

Fig. 4: Three-dimensional view of mitral valve in transesophageal echocardiography showing flail motion of bioprosthetic leaflet
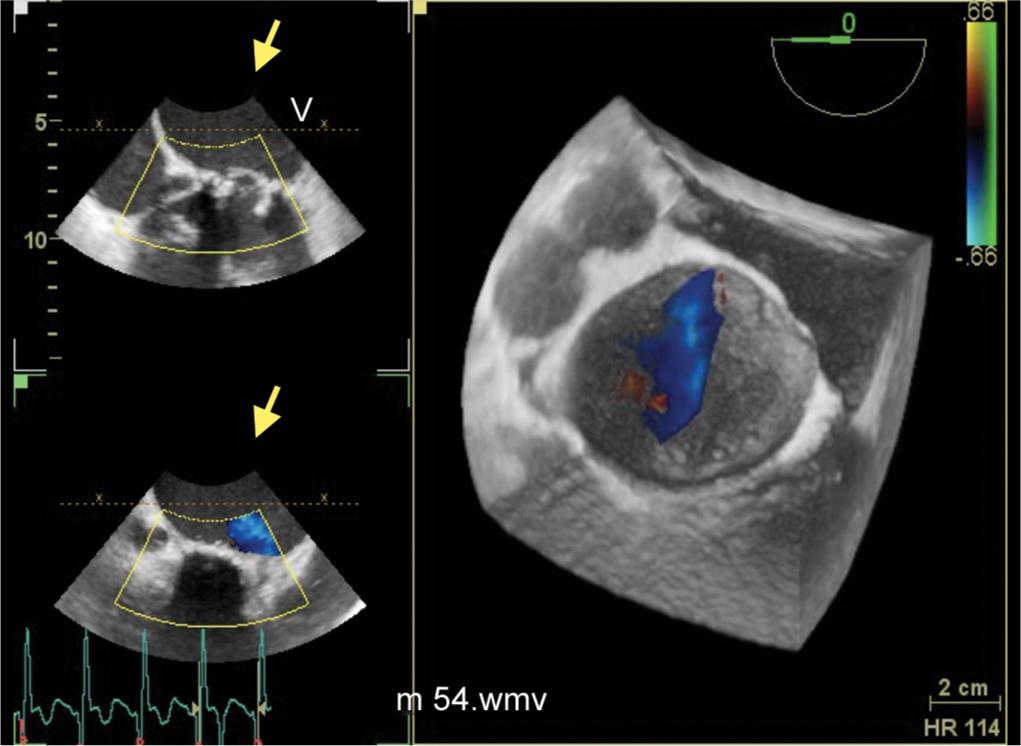
Fig. 5: 3D color Doppler confirms regurgitant jet originating from the torn anterior cusp of the prosthesis

Fig. 6: Extracted bioprosthetic valve showing calcification and leaflet destruction in the ventricular side of valve
DISCUSSION
Bioprosthetic heart valve (BHV) degeneration is slowly progressive in nature with 20 to 30% of the valves becoming dysfunctional within 10 years of implantation.4 Mitral valve is highly prone for wear and tear, especially at the commissural region due to higher closing pressures.5 Other common causes for prosthetic mitral valve regurgitation include pannus formation, valve thrombosis, and IE. Identifying the exact mechanism of regurgitation in BHV is crucial for deciding further management plan. Although TTE remains the initial diagnostic modality of choice, the position of TEE probe just behind mitral valve, presence of omni-plane angle, and lack of acoustic shadowing help in clear appreciation of mechanism of mitral regurgitation in TEE. Moreover, the diagnostic accuracy of TTE is influenced by other factors, such as the presence of pericardial effusion, emphysema, obesity, or prior sternotomy.6
Calcification of BHV commonly causes cusp tear and perforation, which leads to regurgitation. Immune system plays a vital role in the initiation of calcification.7 Transesophageal echocardiography helps in clear visualization of cusp wear and tear. In index case, echo-dense areas in the prosthetic valve were probably due to calcification leading to cusp destruction. Posteriorly directed eccentric jet implied that the tear was in the anteriorly placed prosthetic leaflet and its subsequent flail motion into the left atrium. Morphological examination showed type I tear in the bioprosthetic cusp as cause of severe mitral regurgitation. The cusp lesions are classified into four types based on the morphological studies.8 Type I lesions involve the free edges of the cusps; type II lesions comprise linear perforations that extend along the basal regions of the cusps, forming an arc parallel to the sewing ring; type III lesions involve large, round, or oval perforations that occupy central regions of the cusps; and type IV lesions are small pinhole-like perforations. As the cause of mitral regurgitation in our case was endogenous in nature, redo mitral valve replacement was carried out using CPB.
Another important cause for late prosthetic valve failure causing regurgitation is due to excessive pannus formation. Pannus formation is an exuberant reaction resulting from periannular tissue healing that results in cusps thickening and regurgitation due to restriction of prosthetic valve leaflets motion.9 Sometimes, prosthetic valve thrombosis may also be associated with new-onset regurgitation or mixed stenosis and regurgitation. Distinguishing between thrombus and pannus as a cause of regurgitation is important because thrombus can potentially be treated with fibrinolysis, whereas pannus requires surgical intervention. Thrombus is usually associated with a short history of inadequate anticoagulation. On echocardiography, thrombus appears as a large mobile mass with low echo-density, while a pannus appears as a small immobile echo-dense mass.10,11
Infective endocarditis causes early PVD with incidence of 1–6%.12 Vegetations on prosthetic valves are more difficult to detect by TTE than those involving native valves because of acoustic shadow into left atrium, and therefore, TEE should always be used if the diagnosis of prosthetic endocarditis is suspected. Echocardiographic diagnostic criteria for IE include (1) a mobile echo-dense masses implanted in a valve or mural endocardium in the trajectory of a regurgitant jet or implanted in prosthetic material with no alternative anatomical explanation, (2) presence of abscess, and (3) presence of a new dehiscence of a valvular prosthesis. Transesophageal echocardiography plays a crucial role in identifying the strands in mitral position, suggestive of IE.13 Transesophageal echocardiography has better sensitivity (86–94%) and specificity (88–100%) for diagnosis of IE when compared with TTE (sensitivity 36–69%).13 Transesophageal echocardiography also has higher sensitivity in identifying ring abscesses in the mitral position than TTE (57% vs 9%).14 Vegetations tend to start in the valve ring area and then spread to the leaflets, leading to valve coaptation defects. It may be difficult to differentiate between vegetations and thrombosis on imaging, so decision should be considered in terms of clinical context such as Duke’s clinical criteria.
With advances in real-time 3D technology, it is easy to visualize the prosthetic valve components such as the leaflets, rings, and struts of all prosthetic valves, irrespective of the position. Lack of acoustic shadowing, assessment of ventricular side of mitral valve, provision of surgeon’s view of mitral valve, and calculation of accurate mitral regurgitation effective orifice area are the important benefits of real-time 3D technology. Moreover, 3D TEE can clearly identify valve pannus overgrowth or vegetations.6
CONCLUSION
Transesophageal echocardiography remains to be a useful tool in identifying the exact mechanism for PVD and deciding appropriate management of the patient.
Video 1: Transesophageal echocardiography four-chamber view with color doppler showing mitral regurgitation
Video 2: 3D mitral valve in TEE showing flail motion of bioprosthetic leaflet
Video 3: Midesophageal two-chamber view with colour doppler showing eccentric jet
Video 4: Midesophageal four-chamber view showing flail motion of torn bioprosthetic leaflet
REFERENCES
1. Edmunds LH. Jr. Thromboembolic complications of current cardiac valvular prostheses. Ann Thorac Surg 1982;34(1):96–106. DOI: 10.1016/s0003-4975(10)60862-4.
2. Russhard P, Weerasinghe A. Intermittent jamming of a bileaflet mechanical heart valve in the absence of any extrinsic cause of obstruction. Eur J Echocardiogr 2011;12(3):E19. DOI: 10.1093/ejechocard/jeq173.
3. Ma WG, Hou B, Abdurusul A, et al. Dysfunction of mechanical heart valve prosthesis: experience with surgical management in 48 patients. J Thorac Dis 2015;7(12):2321–2329. DOI: 10.3978/j.issn.2072-1439.2015.12.25.
4. Jamieson W, Munro A, Miyagishima R, et al. Carpentier-edwards standard porcine bioprosthesis: clinical performance to seventeen years. Ann Thorac Surg 1995;60(4):999–1006. DOI: 10.1016/0003-4975(95)00692-e.
5. Butany J, Leask R. The failure modes of biological prosthetic heart valves. J Long Term Eff Med Implants 2001;11(3-4):115–136. DOI: 10.1615/JLongTermEffMedImplants.v11.i34.30.
6. Habets J, Budde RP, Symersky P, et al. Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol 2011;8(8):466–478. DOI: 10.1038/nrcardio.2011.71.
7. Schoen F, Fernandez J, Gonzalez-Lavin L, et al. Causes of failure and pathologic findings in surgically removed inoescu-shiley standard bovine pericardial heart valve bioprostheses: emphasis on progressive structural deterioration. Circulation 1987;76(3):618–627. DOI: 10.1161/01.cir.76.3.618.
8. Ishihara T, Ferrans VJ, Boyce SW, et al. Structure and classification of cuspal tears and perforations in porcine bio-prosthetic cardiac valves implanted in patients. Am J Cardiol 1981;48(4):665. DOI: 10.1016/0002-9149(81)90145-4.
9. Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology 2009;55(2):135–144. DOI: 10.1111/j.1365-2559.2008.03190.x.
10. Barbetseas J, Nagueh SF, Pitsavos C, et al. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol 1998;32(5):1410–1417. DOI: 10.1016/s0735-1097(98)00385-4.
11. Lin SS, Tiong IY, Asher CR, et al. Prediction of thrombus-related mechanical prosthetic valve dysfunction using transesophageal echocardiography. Am J Cardiol 2000;86(10):1097–1101. DOI: 10.1016/s0002-9149(00)01166-8.
12. Edmunds L, Clark R, Cohn L, et al. Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ann Thorac Surg 1996;62(3):932–935. DOI: 10.1016/s0003-4975(96)00531-0.
13. Rozich JD, Edwards WD, Hanna RD, et al. Mechanical prosthetic valve associated strands: pathologic correlates to transesophageal echocardiography. J Am Soc Echocardiogr 2003;16(1):97–100. DOI: 10.1067/mje.2003.36.
14. Evangelista A, Gonzalez-Alujas MT. Echocardiography in infective endocarditis. Heart 2004;90(6):614–617. DOI: 10.1136/hrt.2003.029868.
________________________
© The Author(s). 2019 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.