CASE REPORT | https://doi.org/10.5005/jp-journals-10034-1110 |
Point-of-care Echocardiography in Critically Ill COVID-19 Infected Patients: Will it Change the Management?
1–4,6Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India
5Department of Cardiac Anesthesia, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Corresponding Author: Sunder L Negi, Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India, Phone: +91 9888446388, e-mail: dr.sundernegi@gmail.com
How to cite this article Gourav KP, Negi SL, Biswas I, et al. Point-of-care Echocardiography in Critically Ill COVID-19 Infected Patients: Will it Change the Management? J Perioper Echocardiogr 2020;8(1):6–11.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Personal protective measures make traditional clinical examination difficult in the context of the novel coronavirus-2019 (COVID-19) pandemic. Point-of-care ultrasound and echocardiography may ably complement clinical examination and allow optimal management of COVID-19 as well as coexisting comorbidities. Here, the authors describe a series of cases illustrating the same and discuss how point-of-care echocardiography can be expediently and safely implemented.
Keywords: COVID-19, Chest X-ray, Point-of-care echocardiography.
INTRODUCTION
The novel coronavirus-2019 (COVID-19) pandemic has infected >6,000,000 people worldwide and caused >360,000 deaths. Its high contagion rate mandates routine use of personal protective equipment (PPE) while handling these cases. Fundamental clinical examinations like palpation and auscultation are difficult to perform after wearing a PPE kit.1 Hence, a point-of-care ultrasound and echocardiography may play an essential role in overcoming these limitations and help in the evaluation of these patients. Here, we describe our experience in a few critically ill COVID-19 patients, where echocardiography played an important role in their management. Written informed consent was obtained from all subjects.
CASE DESCRIPTIONS
Case 1
A 79-year-old woman with a COVID-19 infection was brought to our hospital with the Medical Research Council (MRC) grade III dyspnea. Though she had a history of shortness of breath on mild exertion for 2 years, she had never undergone any cardiac evaluation. On examination, she had a heart rate of 110/minutes with a respiratory rate (RR) of 42/minutes and had a room air saturation of 82%. She was kept on a nasal prong with an oxygen (O2) flow of 5 L/minute. Severe aortic stenosis (AS) with a mean gradient of 64 mm Hg was diagnosed by transthoracic echocardiography (TTE) with a left ventricular (LV) ejection fraction (EF) of 30% (Fig. 1 and Video 1). The ascending aorta (AA) was also dilated (46 mm). A diuretic and a beta-blocker were started in view of severe AS, low LV EF, and dilated AA, in addition to COVID-19 management. Her heart rate decreased gradually, reaching 65/minutes on day 4. Her R/R and O2 requirement become normalized by the 5th day of the intensive care unit (ICU) stay. She was discharged from the hospital after negative nasopharyngeal swabs on day 19 and is being planned for aortic valve and AA intervention on an elective basis.
Case 2
A 34-year-old male patient of COVID-19 presented with MRC grade IV dyspnea, room air saturation of 81%, and a RR of 49/minutes. Two years back, he was diagnosed with grade V chronic kidney disease (CKD) and advised maintenance hemodialysis (HD) twice a week. However, he missed dialysis for some time in the context of self-imposed isolation due to pandemic and gradually developed breathlessness. He was hemodynamically stable but agitated on presentation. Chest X-ray was congruent with COVID-19 with bi-basal infiltrates and diffuse opacities. TTE revealed a normal biventricular function with moderate pericardial effusion not causing a diastolic collapse of the right atrium and right ventricle (Figs 2A and B and Videos 2 and 3). Besides, a massive pleural effusion (PE) of 7.2 cm and moderate PE of 3.6 cm was found in right and left pleural cavities, respectively (Figs 2C and D and Videos 4 and 5). His respiratory distress decreased after the removal of 900 mL fluid from the right pleural cavity. On the same day, HD was performed with removal of 1,500 mL ultrafiltrate, followed by HD on every alternate day. His RR and O2 requirement gradually normalized, achieving a room air SPO2 of 97% on day 7 of ICU stay.
Case 3
A 68 year-old-female patient was referred to our hospital with unstable hemodynamics. She was a known case of grade V CKD requiring HD twice a week. Unfortunately, she missed the HD for some time due to the COVID-19 pandemic and become delirious at home. She was taken to a local hospital with worsening signs and symptoms after refusing feeds for 3 days. There she was also found to be infected with COVID-19. An HD was planned at a local hospital, however, it was terminated within 1 hour due to hemodynamic instability and started on infusion noradrenaline (NA) at 0.1 μ/kg/minute and referred to our hospital. On examination, she was delirious and all her peripheral veins were collapsed. TTE revealed a normal cardiac function with low left ventricular end-diastolic volume (LVEDV) indicating hypovolemia (Fig. 3A and Video 6). Further examination revealed a small inferior vena cava (IVC) and a highly collapsible internal jugular vein (IJV) (Figs 3B and C and Videos 7 and 8). Despite being grade V CKD, a fluid challenge was given under the guidance of TTE. Her hemodynamics became stabilized, allowing tapering of the vasopressor. Later she underwent HD without removal of any ultrafiltrate and she maintained stable hemodynamics throughout the procedure.
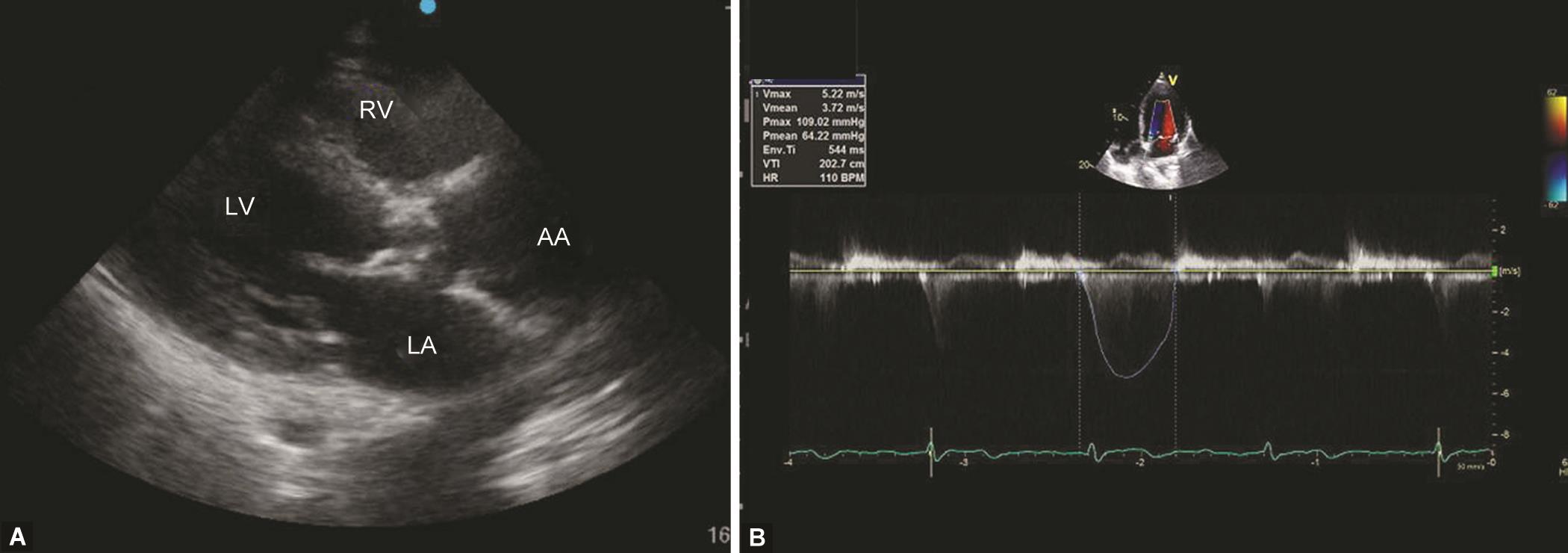
Figs 1A and B: (A) A parasternal long-axis view shows a thickened aortic valve with dilated ascending aorta; (B) A continuous wave placed over the aortic valve in 5-chamber view revealed a gradient of 64 mm Hg. AA, ascending aorta; LA, left atrium; LV, left ventricle; RV, right ventricle
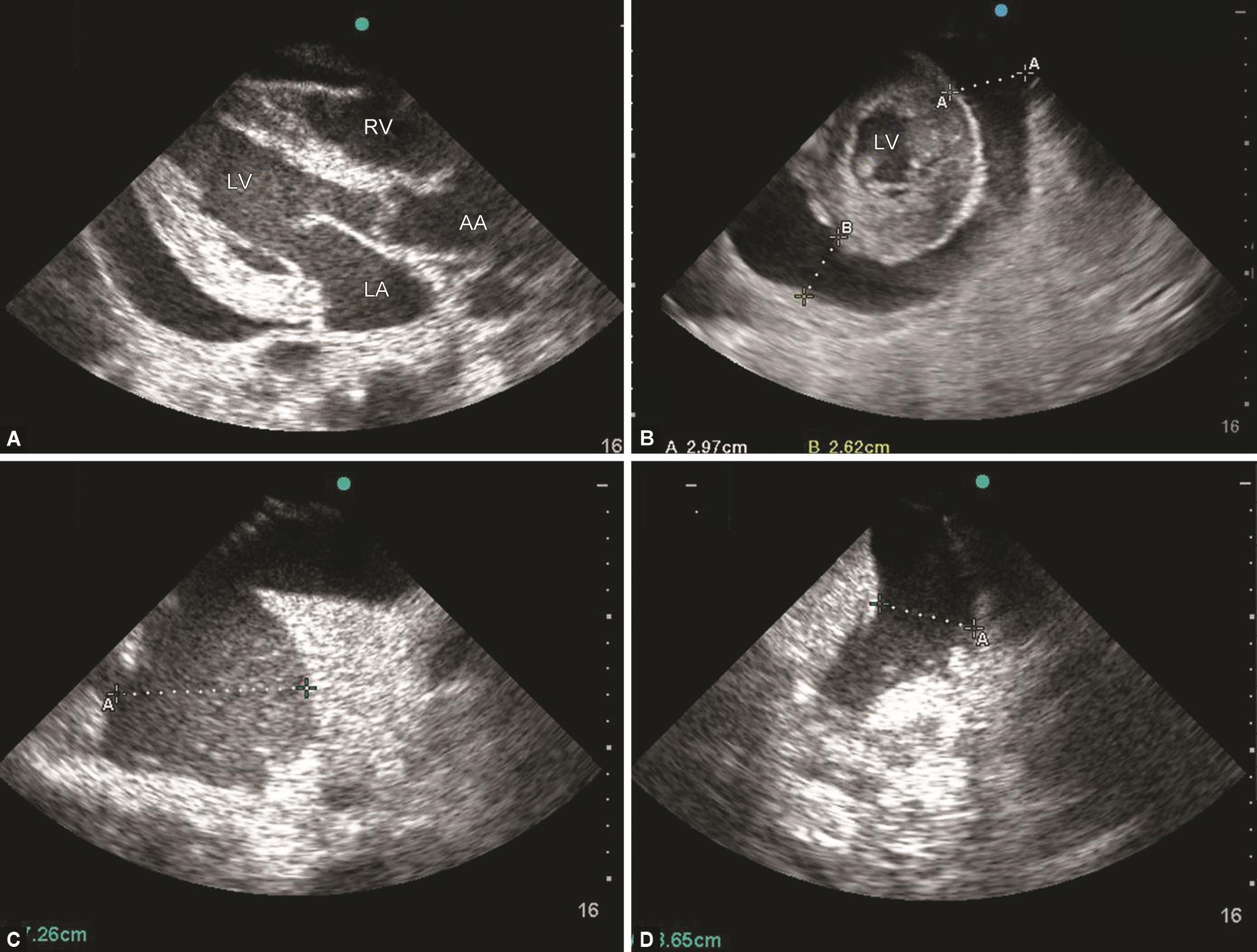
Figs 2A to D: A) A parasternal long-axis view and (B) a midpapillary short-axis view show a moderate pericardial pleural effusion. A lung ultrasound shows, (C) a right-sided massive pleural effusion, and (D) a left-sided moderate pleural effusion with an underlying collapsed lung. AA, ascending aorta; LA, left atrium; LV, left ventricle; RV, right ventricle

Figs 3A to D: (A) A mid-papillary short-axis view shows a good contractility and low left ventricular end-diastolic volume—the “kissing papillary sign” of hypovolemia; (B) Subcostal inferior vena cava view shows a small and collapsible inferior vena cava (IVC) with quiet respiration; (C) A highly collapsing internal jugular vein (IJV) seen in cross-sectional view with quiet respiration confirming hypovolemia; (D) M-mode placed over IVC shows a respiratory variation. CA, carotid artery; LV, left ventricle; RV, right ventricle
Case 4
A 42-year-old male patient diagnosed with COVID-19 was referred to our hospital with unstable hemodynamics with support of NA 0.12 μ/kg/minute. Previously, he was taken to a local hospital and kept on nasal prongs due to low oxygen saturation. In view of bilateral chest infiltrates a restricted fluid strategy was adopted. Later he developed hemodynamic instability and referred to our hospital. TTE examination revealed a small and collapsible IVC with a low LVEDV similar to case 3 suggesting hypovolemia (Fig. 3D). His hemodynamics became stabilized after a fluid challenge and the NA was tapered.
Case 5
A 60-year-old asymptomatic male patient found infected with COVID-19 was kept in an isolation ward at our hospital. Three days later, he developed chest pain with elevated troponin levels without any signs of ST-elevation on electrocardiography (ECG). He became stable after conservative management with low molecular weight heparin and dual antiplatelet therapy. Four days later, he again developed chest pain associated with unstable hemodynamics and ST elevation in the anterior chest leads on ECG. A newly developed regional wall motion abnormality (RWMA) was confirmed in the anteroseptal area of the LV on the TTE examination, confirming myocardial infarction (MI) (Fig. 4 and Video 9). He was thrombolyzed with streptokinase leading to symptomatic and hemodynamic improvement. He had an uneventful ICU stay after that episode.
Case 6
A 30-year-old male patient suffering from grade V, CKD was brought to our hospital with MRC grade IV dyspnea. He had an R/R of 52/minutes with an SPO2 of 84% on 100% O2 even after 1 cycle of HD. Chest X-ray revealed diffuse patchy infiltrates along with some lower lobe consolidation. TTE revealed a normal right and left ventricular function with moderate tricuspid regurgitation (TR) (Fig. 5A and Video 10). Raised pulmonary artery (PA) pressure could be diagnosed from the TR jet (Fig. 5B). In view of respiratory distress, the patient was intubated and kept on mechanical ventilation (MV). TTE done 6 hours later found a decrease in PA pressures. However, his lung compliance gradually deteriorated and we lost the patient on day 7.
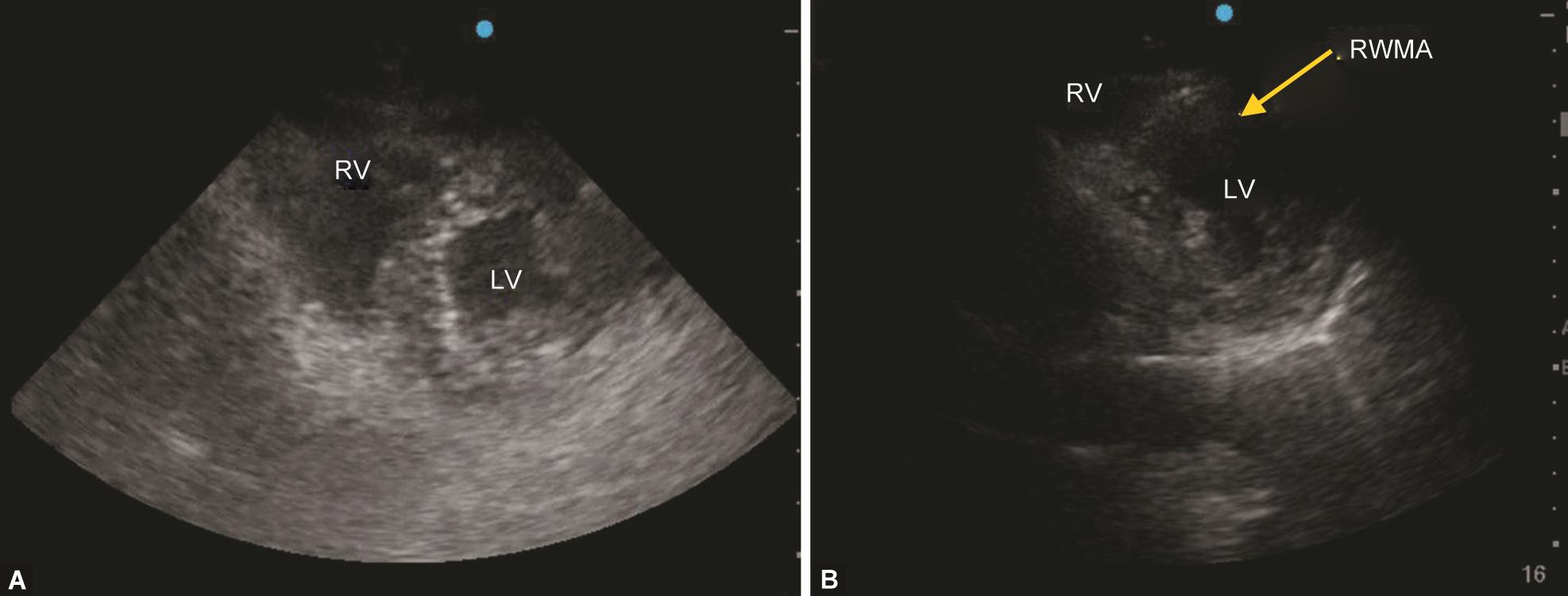
Figs 4A and B:: (A) A mid-papillary short-axis view shows a normal cardiac function before the development of ST-elevation myocardial infarction; (B) A newly developed regional wall motion abnormality (RWMA) in the anteroseptal area was observed after the onset of chest pain with suggestive ECG changes. LV, left ventricle; RV, right ventricle

Figs 5A and B:: (A) A parasternal long-axis right ventricular inflow view shows a moderate tricuspid regurgitation (TR); (B) A continuous wave was placed over the TR in 4-chamber view. RA, right atrium; RV, right ventricle
Case 7
A 20-year-old female patient was kept in an isolation ward at our hospital after a COVID-19 infection. She was asymptomatic till day 5 and suddenly developed MRC grade IV dyspnea. She was immediately shifted to ICU and a TTE examination was done to rule out pulmonary thromboembolism or associated cardiac abnormality. A normal cardiac function without any cardiac abnormality was found on TTE. Chest X-ray and investigations done later were also normal. Her dyspnea gradually decreased after the results of her investigations were communicated to her. On thorough history, a diagnosis of a panic attack was made and she was asymptomatic throughout the hospital stay after proper counseling.
The summary of the cases and their management based on TTE findings were described.
DISCUSSION
COVID-19 has a wide spectrum of presentations ranging from mild self-limiting respiratory tract illness to severe acute respiratory distress, multiple organ failure, and death.2 Hypoxemic respiratory failure and hypotension requiring vasopressor support are the two most common reasons for ICU admissions in these patients. The lung is the most common organ infected by COVID-19 causing hypoxia.3 However, hypoxia developed due to undiagnosed preexistence disease can either mimic or aggravate COVID-19 related hypoxia. A timely diagnosis of those conditions can modify the line of management and help in early recovery. In the index case series, AS with elevated left atrial pressure and a PE were additional contributing factors for hypoxia in cases 1 and 2, respectively. In both cases, TTE played an important role in timely identification and management.
Hypovolemia is the most common correctable cause of unstable hemodynamics. Due to improper assessment vasopressors are sometimes administered instead of volume to improve the blood pressure. These scenarios are commonly encountered while managing patients with CKD and acute respiratory distress syndrome. In view of hypoxia and chest infiltrates on X-ray, pre-hospital fluid resuscitation is rarely performed in patients with COVID-19 which can lead to hemodynamic instability and sometimes even can develop pre-renal acute kidney injury.4,5 In cases 3 and 4, echocardiography played a crucial role in guiding fluid resuscitation and also helped in assessing fluid responsiveness by looking at the LVEDV, IVC, and IJV. Pitfalls of various echocardiographic methods such as work of breathing, mechanical ventilation, arrhythmias, and presence of TR should always keep in mind while assessing volume status.6 Sometimes, IVC can move out of the plane of the ultrasound beam with respiration, falsely mimicking changes in diameter leading to misinterpretation.6 As superior vena cava (SVC) is difficult to see with TTE, the SVC collapsibility index (CI), can be replaced with IJV CI as a second-line alternative site for IVC-CI in whom visualization of the latter is difficult.7 In addition, it took less time to acquire IJV-CI compared to IVC-CI measurement.
Myocardial injury in COVID-19 infected patients can occur due to multiple reasons like plaque rupture, coronary spasm, hypoxic injury, cytokine storm, micro-thrombi, or due to direct endothelial or vascular injury. STEMI is the most common clinical manifestation in COVID-19 patients, but these are associated with an obstructive lesion in only 60% of the cases, creating a management dilemma.8 A STEMI associated with hemodynamic instability and newly developed RWMA in an anteroseptal area on TTE developed in case 5, lead us to start a thrombolytic therapy.
An increase in pulmonary vascular resistance caused by hypoxia, hypercarbia, and inflammation in case 6 was diagnosed with the help of TR jet velocity in TTE. Knowledge of right ventricular dysfunction by TTE provides valuable information related to circulatory and respiratory management strategies in patients with COVID-19 ARDS.9 Severely ill COVID-19 patients can develop pulmonary embolus as it is associated with high coagulopathy.10 Echocardiography helps in ruling out cardiac causes for sudden onset of respiratory distress as in case 7, where the patient developed a panic attack after 5 days of isolation.
Echocardiography has a clear advantage over the other diagnostic modality due to its portability. Hence, patients need not move from their place for imaging, thus preventing the risk of virus transmission in the clinic or the hospital area. However, due to its high contagion rate, healthcare workers will be at additional risk while doing echocardiography exams as they come in close contact with COVID-19 patients. This limitation can be overcome by doing rapid scanning to avoid unwanted exposure. Few suggestions from our experience to decrease COVID-19 exposure are:
- To be performed by a trained and experienced echocardiographer for rapid scanning.
- Well orientation of knobology before examining to avoid unnecessary time lag as the orientation of keys are different in different machines.
- Use a high-resolution echocardiography machine to obtain a clear image.
- To examine the left lateral position while sitting right to the patient for optimal imaging as well as to decrease direct contact.
- Acquire minimum and important views to get maximum information.
- Use offline methods to perform hemodynamic calculations.
In conclusion, the presentation of severe COVID-19 may be coincident with and complicated by various comorbidities, which are themselves risk factors for disease severity. The physical and attitude barriers placed by the contagiousness of the disease may impede evaluation, leading to adverse outcomes. In these scenarios, point-of-care echocardiography can act as a valuable adjunct to clinical management for rapid screening of circulatory and respiratory status to identify various types of shocks and any preexisting cardiac lesions and thus guide for optimal management.
- Video 1: A parasternal long-axis view shows a thickened aortic valve with dilated ascending aorta
- Video 2: A parasternal long-axis view shows a moderate pericardial and pleural effusion
- Video 3: A mid-papillary short-axis view shows a normal left ventricular contractility with moderate pericardial effusion
- Video 4: Lung ultrasound shows a right-sided massive pleural effusion with an underlying collapsed lung
- Video 5: Lung ultrasound shows left-sided moderate pleural effusion with an underlying collapsed lung
- Video 6: A mid-papillary short-axis view shows a good contractility and low left ventricular end-diastolic volume—the “kissing papillary sign” of hypovolemia
- Video 7: Sub-costal view shows a small and collapsible inferior vena cava with quiet respiration
- Video 8: Cross-sectional view of the internal jugular vein shows collapsibility with quiet respiration
- Video 9: A mid-papillary short-axis view demonstrates a regional wall motion abnormality in the anteroseptal area
- Video 10: A color flow Doppler placed in parasternal long-axis view right ventricular inflow view demonstrates a moderate tricuspid regurgitation
REFERENCES
1. Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 2020. DOI: 10.1016/j.jare.2020.03.005.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. DOI: 10.1016/S0140-6736(20)30566-3.
3. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol 2020. 108427. DOI: 10.1016/j.clim.2020.108427.
4. Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respirat Med 2020;8(7):P738–742. DOI: 10.1016/S2213-2600(20)30229-0.
5. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394(10212):1949–1964. DOI: 10.1016/S0140-6736(19)32563-2.
6. Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract 2016;3(2):G1–G2. DOI: 10.1530/ERP-16-0008.
7. Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thora Med 2015;10(1):44. DOI: 10.4103/1817-1737.146872.
8. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation 2020; Apr30.
9. Peng Q, Wang X, Zhang L. Using echocardiography to guide the treatment of novel coronavirus pneumonia. Crit Care 2020;24(1):143. DOI: 10.1186/s13054-020-02856-z.
10. Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. DOI: 10.1148/radiol.2020201544.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.