CASE REPORT | https://doi.org/10.5005/jp-journals-10034-1112 |
Transvalvular Regurgitation across a Tilting Disk Prosthetic Mitral Valve: Role of Intraoperative Real-time Three-dimensional Transesophageal Echocardiography
1–5Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India
6Department of Cardiac Surgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Corresponding Author: Subhrashis Guha Niyogi, Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India, Phone: +91 9748870809, e-mail: 09renol@gmail.com
How to cite this article Niyogi SG, Singh A, Gowda N, et al. Transvalvular Regurgitation across a Tilting Disk Prosthetic Mitral Valve: Role of Intraoperative Real-time Three-dimensional Transesophageal Echocardiography. J Perioper Echocardiogr 2020;8(1):15–17.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Prosthetic valve regurgitation in the immediate post-implantation period can be caused by paravalvular or transvalvular leakage. Close examination with two-dimensional (2D) as well as three-dimensional (3D) transesophageal echocardiography (TEE) is necessary for its proper diagnosis. A case of transvalvular regurgitation across a tilting disk prosthetic valve in mitral position is described here, along with the role of 3D TEE in its diagnosis. Image acquisition and optimization for 3D TEE in prosthetic valve evaluation is also briefly reviewed.
Keywords: 3D echocardiography, Chitra heart valve prosthesis, Prosthetic valve dysfunction.
BACKGROUND
Prosthetic valve implantation is a common intervention for valvular heart disease. Cautious immediate post-bypass evaluation screens for any residual defect and dysfunction to achieve optimum results. This is helpful in avoiding any significant long-term morbidity.
CASE DESCRIPTION
A 55-year-old woman presented with progressive dyspnea on exertion, increasing to New York Heart Association grade III over 3 years, and palpitation for 3 months. Initial evaluation showed irregularly irregular rhythm with a pulse rate of 76 and pulse deficit of 12. Transthoracic echocardiography showed dilated left atrium and ventricle with mild left ventricular dysfunction, mild pulmonary hypertension (peak tricuspid regurgitation gradient of 25 mm Hg at a central venous pressure of 8 mm Hg), and thickening of the mitral valves and subvalvular apparatus suggestive of rheumatic heart disease. Color flow and Doppler imaging showed severe eccentric mitral regurgitation, severe mitral stenosis with a peak gradient of 26 mm Hg and mean gradient of 12 mm Hg, mild aortic regurgitation, and mild tricuspid regurgitation.
After further workup, she underwent mitral valve replacement with a #26 tilting disk prosthesis (TTK Chitra heart valve prosthesis) in the mitral anatomical position through the trans-left atrial route. The initial intraoperative transesophageal echocardiographic (TEE) assessment following cardiopulmonary bypass (CPB) showed an eccentric posterolaterally directed regurgitant jet in the left atrium which seemed more than the usual washing jets. This had a dense parabolic spectral Doppler waveform with a peak velocity of 5 m/s and a vena contracta width of 4.5 mm. A thorough two-dimensional (2D) examination in multiple views revealed the origin of the jet to be inside the prosthetic valve sewing ring (Fig. 1 and Video 1). This excluded a paravalvular leak. Three-dimensional (3D) echocardiography with color Doppler confirmed this, demonstrating the sewing ring and the regurgitant jet originating inside (Fig. 2 and Video 2). An optimized 3D image also demonstrated an echogenic mass in the 6 o’clock position of the valve on the left atrial side (Fig. 3 and Video 3).
The patient underwent a second run of CPB where prolapsing chordae of the posterior mitral leaflet were found to obstruct the complete closure of the prosthetic valve. These were excised to allow normal prosthetic valve closure. After separation from CPB, the TEE showed a decreased regurgitant jet and normal leaflet motion. This was further confirmed on 3D TEE (Fig. 4 and Video 4).
DISCUSSION
Transesophageal echocardiography remains the primary modality of prosthetic valve assessment in the immediate post-CPB period, allowing immediate revision.1 The sensitivity and specificity of TEE for paravalvular leaks has been found to be better than TTE and comparable to cardiac computed tomography.2 This avoids the cost and morbidity as well as the hemodynamic insult of a second surgery and is always recommended, barring any contraindications.3 The TEE assessment is made difficult by acoustic shielding and shadowing by prosthetic valve material or sewing ring and multiple views are required to confirm valve leaflet or occluder morphology and mobility, check the integrity and stability of the sewing ring and identify the presence of any abnormal structures on any components of the prosthesis. Unstable hemodynamics, elevated cardiac output, and potentially elevated post-CPB pre-valve velocities also complicate Doppler hemodynamic assessment.
The 3D echocardiography offers incremental benefits over 2D in evaluating prosthesis function, identifying and characterizing regurgitant jets, prosthesis components (occluders, rings, and struts), and any thrombus, pannus, and dehiscence.
The current American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines advocate comprehensive examination in multiple 2D and 3D views as well as Doppler parameters for an integrative assessment and quantification for prosthetic valve regurgitation.1,4
A certain amount of physiological regurgitation is expected in prosthetic valves. For mechanical prostheses, this can be created by the closing occluders (closing volume) or leakage through valve components (leakage volume). These jets are supposed to prevent valve stasis and thrombosis and hence are called “washing jets”. The patterns of these jets are characteristic of the specific valve designs. Bileaflet prostheses have two central and two peripheral washing jets, while monoleaflet tilting disk prostheses have a large central and two smaller peripheral washing jets. Bioprosthetic prostheses on the other hand may have small central or commissural regurgitant jets. These physiological jets usually have a narrow origin, symmetrical pattern, and low momentum which is reflected in homogeneous laminar color flow patterns.
The goal of intraoperative echocardiography is to differentiate these physiological jets from pathological jets. Pathological jets can be central, i.e., transvalvular, and paravalvular in origin. Echocardiographic signs include a wider origin turbulent regurgitant jet with increased anterograde and retrograde velocities, dense spectral Doppler waveform, and formation of proximal isovelocity surface area on the ventricular side. Differentiation of transvalvular and paravalvular regurgitation requires examination for the origin of the jet in 2D or 3D echocardiography.
Paravalvular jets originate outside the sewing ring and are a due to tissue, surgical technical factors, or infective endocarditis in the postoperative period. The echocardiographic hallmark is a jet originating outside the sewing ring, readily apparent on both 2D and 3D examination. The 3D examination in addition may also show a defect.5
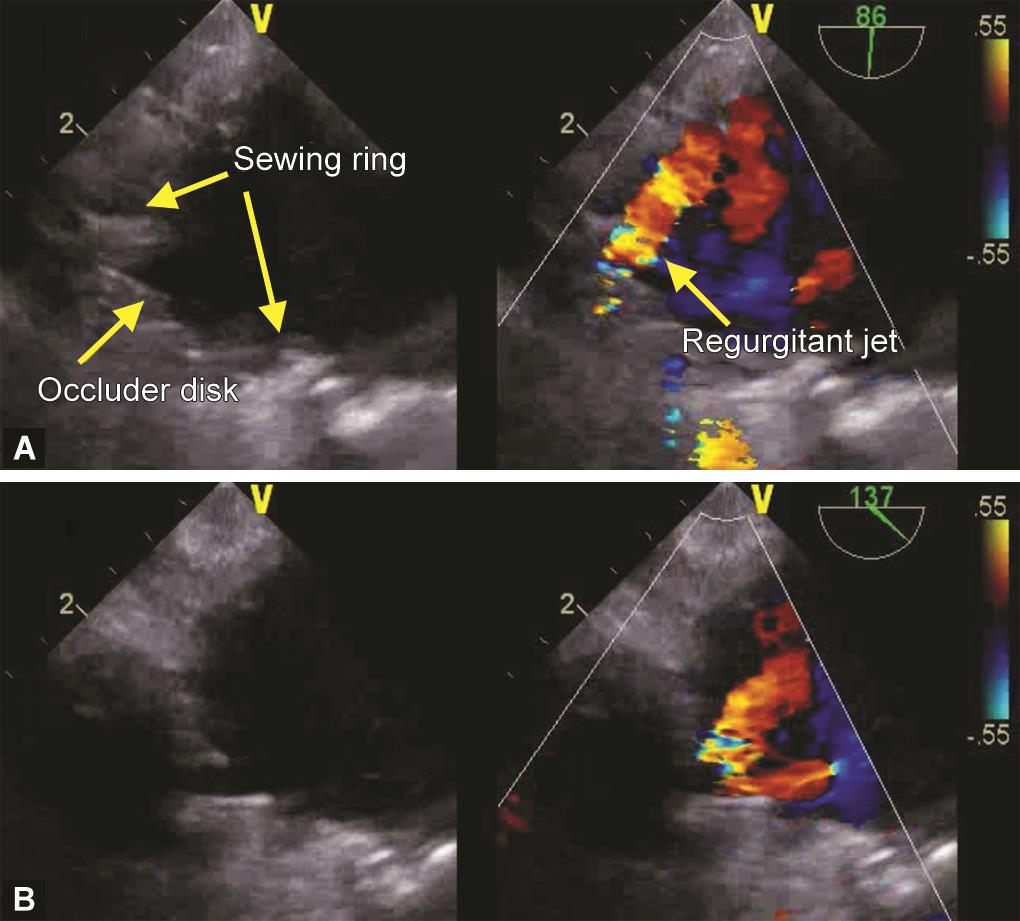
Figs 1A and B: Intraoperative transesophageal mid-esophageal two-chamber (A) and long-axis (B) views showing a tilting disk mechanical prosthetic valve in mitral position with a regurgitant jet originating inside the sewing ring
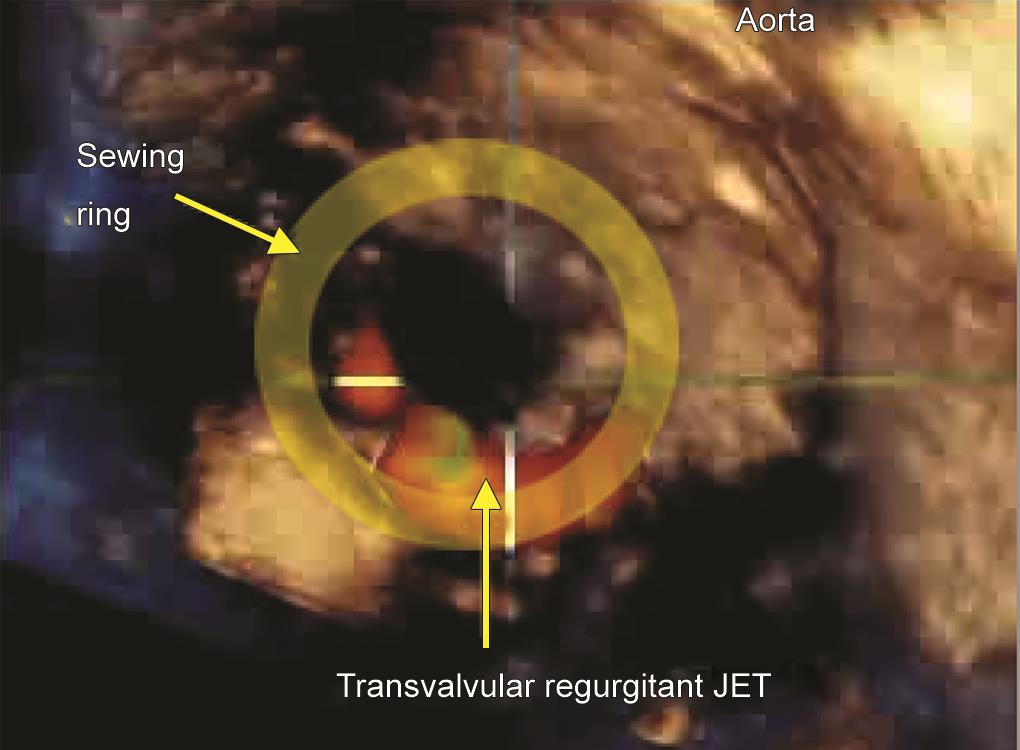
Fig. 2: Intraoperative transesophageal 3D “surgeon’s view” with color Doppler demonstrating a regurgitant jet in the posterior aspect after implantation of a tilting disk prosthetic heart valve

Figs 3A and B: Intraoperative transesophageal 3D “surgeon’s view” of a tilting disk prosthetic heart valve in closed (A) and open (B) position, showing a mass at the 6 o’clock position. Surgical examination showed it to be a prolapsing chordae fragment obstructing valve closure and causing regurgitation
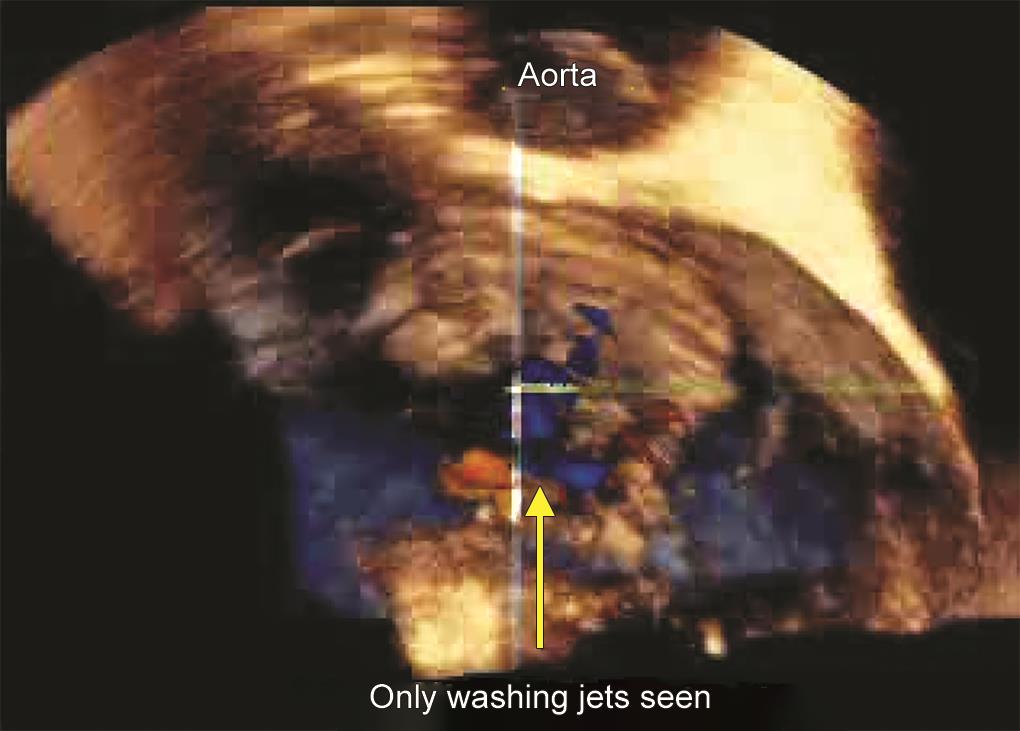
Fig. 4: Intraoperative transesophageal 3D “surgeon’s view” with color Doppler demonstrating reduced regurgitant jet and only visible washing jets after revision, with excision of prolapsing chordae fragments
Central jets originate from degenerated or destroyed bioprosthetic valves or from interference in the closure of mechanical valves.6 For tilting disk valves, central regurgitation due to prolapsing parts of the subvalvular apparatus has been described.7 In our case, this could be identified on the 2D echo from the origin of the suspected jet between the occluder and the sewing ring, but not outside. This was further demonstrated by 3D echocardiography. A single-center trial demonstrated superiority in identification and characterization of suspected paravalvular leaks using real-time 3D TEE.8 Transvalvular jets are often eccentric and harder to characterize, and 3D echocardiography with color flow imaging has a definite advantage in these cases.
For 3D imaging, the best imaging planes are located and optimized using 2D imaging. After that, acquisition in full volume or 3D zoom mode, with and without color Doppler can be done over a single beat or over multi-beat with ECG gating. With a prosthetic valve in the mitral position, the acquisition is usually in mid-esophageal four chamber view, providing the left atrial perspective and avoiding any acoustic shadowing. If available, the transgastric view allows better visualization from the left ventricular perspective. This can demonstrate subvalvular pathology like the current case. Balancing spatial and temporal resolution is important. Maximum possible cropping can be done at acquisition, and in presence of a stable rhythm, multi-beat imaging can be used to optimize the frame rate as well as resolution. 3D and color gain are optimized, and only anterograde or retrograde color flow can be displayed for clarity. After the acquisition, the dataset can be optimized and cropped for the best visualization. Conventionally, 3D images from the mitral valve are presented in the “surgeons’ view”, i.e., the left atrial perspective. This needs rotation of the cropped view to position the aortic valve at 12 o’clock and the left atrial appendage at 9 o’clock. Close inspection of the image can then show any structural abnormalities or regurgitant jets.8,9
CONCLUSION
Real-time 3D TEE allowed superior detection and improved characterization of transvalvular regurgitation in our patient. Timely reintervention ensured a good outcome. 3D echocardiography can be a valuable addition to 2D echocardiography for the assessment of prosthetic heart valves.
Written informed consent was provided by the patient for publication of this case report.
- Video 1: Intraoperative transesophageal mid-esophageal two-chamber (A) and long-axis (B) views showing a tilting disk mechanical prosthetic valve in mitral position with a regurgitant jet originating inside the sewing ring
- Video 2: Intraoperative transesophageal 3D “surgeon’s view” with color Doppler demonstrating a pansystolic regurgitant jet in the posterior aspect after implantation of a tilting disk prosthetic heart valve
- Video 3: Intraoperative transesophageal 3D “surgeon’s view” of a tilting disk prosthetic heart valve, showing a mass at the 6 o’clock position (marked by the arrow). Surgical examination showed it to be a prolapsing chordae fragment obstructing valve closure and causing regurgitation
- Video 4: Intraoperative transesophageal 3D “surgeon’s view” with color Doppler demonstrating reduced regurgitant jet and only visible washing jets after revision, with excision of prolapsing chordae fragments. The washing jets are transient, compared to the pansystolic pathological regurgitant jet
REFERENCES
1. Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves. Eur Hear J – Cardiovasc Imag 2016;17(6):589–590. DOI: 10.1093/ehjci/jew025.
2. Suh YJ, Hong G-R, Han K, et al. Assessment of mitral paravalvular leakage after mitral valve replacement using cardiac computed tomography. Circ Cardiovasc Imag 2016;9(6):e004153.
3. Thys DM, Abel MD, Brooker RF, et al. Practice guidelines for perioperative transesophageal echocardiography. Anesthesiology 2010;112(5):1084–1096. DOI: 10.1097/ALN.0b013e3181c51e90.
4. Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound. J Am Soc Echocardiogr 2009;22(9):975–1014. DOI: 10.1016/j.echo.2009.07.013.
5. Singh S, Hasija S, Chauhan S. Paravalvular leak after mitral valve replacement: advantage of 3D echo. Ann Card Anaesth 2014;17(2):137. DOI: 10.4103/0971-9784.129855.
6. Pang PYK, Garwood S, Hashim SW. Intraoperative bioprosthetic valve dysfunction causing severe mitral regurgitation. Ann Thorac Surg 2017;103(4):e317–e319. DOI: 10.1016/j.athoracsur.2016.09.019.
7. Dinesh Kumar U, Nareppa U, et al. Transvalvular mitral regurgitation following mitral valve replacement a diagnostic dilemma. Ann Card Anaesth 2015;18(4):584. DOI: 10.4103/0971-9784.166476.
8. Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25(1):3–46. DOI: 10.1016/j.echo.2011.11.010.
9. Chaudhari S, Prajapati J, Shastri N, et al. Evaluation of prosthetic valve dysfunction by three-dimensional echocardiography. Hear India 2018;6(2):54. DOI: 10.4103/heartindia.heartindia_1_18.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.